Volume 1, Issue 1 (October 2017)
AOH 2017, 1(1): 29-34 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bahrami A. Design and Evaluation of a Test Atmosphere System for Passive Samplers Development. AOH 2017; 1 (1) :29-34
URL: http://aoh.ssu.ac.ir/article-1-29-en.html
URL: http://aoh.ssu.ac.ir/article-1-29-en.html
Department of Occupational Health, Research Centre for Health Sciences, Faculty of Public Health, Hamedan University of Medical Sciences, Hamadan, Iran • , ar167@yahoo.com
Keywords: Test Atmosphere Generation, Passive Samplers, Solid Phase Microextraction, Exposure Chamber, Air Pollution
Full-Text [PDF 1681 kb]
(1004 Downloads)
| Abstract (HTML) (3025 Views)

Full-Text: (1696 Views)
Design and Evaluation of a Test Atmosphere System for Passive Samplers Development
Abdulrahman Bahrami1*
1 Department of Occupational Health, Research Centre for Health Sciences, Faculty of Public Health, Hamedan University of Medical Sciences, Hamadan, Iran •Corresponding Author: Abdulrahman Bahrami, Email: ar167@yahoo.com, Tel: +98-353-1492197
Abstract
Background: Preparation of the test atmosphere is a crucial step in occupational health, toxicology, and analytical chemistry researches. Methods: A simple and efficient system was developed for preparation of standard test atmosphere for application in occupational and environmental exposure studies, especially passive sampler's validation. Results: The system applicability was tested in various temperature, humidity, and velocity conditions. Results showed that for testing the adsorbent samplers, the system should be in hybrid mode of dilution. Conclusion: The repeatability of the measurements in the proposed system was also examined that was in acceptable range. With double dilution system it is possible to produce concentrations less than part per million.
Keywords: Air pollution; Environmental Monitoring; Solid Phase Microextraction; Toxicology
Introduction
Abdulrahman Bahrami1*
1 Department of Occupational Health, Research Centre for Health Sciences, Faculty of Public Health, Hamedan University of Medical Sciences, Hamadan, Iran •Corresponding Author: Abdulrahman Bahrami, Email: ar167@yahoo.com, Tel: +98-353-1492197
Abstract
Background: Preparation of the test atmosphere is a crucial step in occupational health, toxicology, and analytical chemistry researches. Methods: A simple and efficient system was developed for preparation of standard test atmosphere for application in occupational and environmental exposure studies, especially passive sampler's validation. Results: The system applicability was tested in various temperature, humidity, and velocity conditions. Results showed that for testing the adsorbent samplers, the system should be in hybrid mode of dilution. Conclusion: The repeatability of the measurements in the proposed system was also examined that was in acceptable range. With double dilution system it is possible to produce concentrations less than part per million.
Keywords: Air pollution; Environmental Monitoring; Solid Phase Microextraction; Toxicology
Introduction
Preparation of test atmosphere is a crucial step in development of test methods, toxicological studies, and sampler performance evaluation in occupational and environmental health, toxicology, and analytical chemistry researches. So far, several methods have been used for preparation of test atmosphere1-4. Each of them focused on specific parameters and criteria in design and construction of the systems. Preparation of test atmosphere is vital in occupational hygiene studies to conduct toxicological studies (inhalational dosing of animals), develop and evaluate the exposure assessment methods, test respiratory protection equipment, test air pollution control media, and measure their efficiency.
There are several techniques for preparation of test atmosphere in occupational exposure assessment studies. In general, these techniques can be divided into two main dynamic and static categories.1 However, there are numerous configurations which merge these techniques for generation of test atmosphere in specific cases.2 These techniques are reviewed extensively in the literature.3-5 Static techniques use a specified mass of analyte in a specific volume of a container for generation of test atmospheres. These methods are easy to prepare and cheap. But they also have some disadvantages such as: 1) adsorption of the analyte on the container walls, 2) non- continuous flow preparation, and 3) decrease of concentration due to withdrawal of a specific volume of mixture. Despite all of these negative points, it could be a good method in the case of using large containers and withdrawal of a small portion of this volume (less than 5%). Dynamic techniques deliver continuous mass of analyte to the continuous flow of diluent gas. These techniques can be divided into three general sub-groups of syringe injection, permeation tubes, and gas dilution.
Most techniques reviewed in different papers only described methods for preparation of specific concentration and did not deal with preparation of standard concentration at different environmental conditions. Therefore, preparation of a correct configuration for generating specific concentration of analyte in specific environmental conditions is another problem which must be taken into account. Selection of an appropriate method for generation of test atmosphere in occupational health studies is also another challenge and depends on the target concentration, nature of analyte, desirable environmental parameters, and the purpose of the study. Besides these, a good system must be qualified in other aspects, such as ability to generate a stable concentration in a prolonged time, and produce the desirable concentration in the various environmental conditions.
In this study, a hybrid system based on syringe injection and double dilution (stream dilution) for generation of test atmosphere was desired. The system configuration was set up for development of sampling methods based on passive samplers especially solid phase micro extraction (SPME).
Methods
Chemicals and materials
Carbon tetrachloride (99.5%), 1-butanol (99.5%), tetrachloroethylene (99%), and carbon disulfide (99%) were obtained from Merck (Darmstadt, Germany). USP grade Halothane was purchased from Nicholas Primal (Mumbai, India). SPME fibers included 100 µm poly-dimethylsiloxane (PDMS) and 75 µm carboxen/PDMS (CAR/PDMS) and manual holders were supplied from Supelco (Bellefonte, PA, USA).
Analysis
All new SPME fibers were conditioned in gas chromatography (GC) injector and desorbed according to the procedure described elsewhere.6 All SPME samples were analyzed with a Varian 3800 GC equipped with a Saturn 2200 ion trap mass spectrometer (MS). The GC was fitted with a 60 m×0.25 mm I.D and 1.5 μm film thickness capillary VOCOL column (Supelco, Bellefonte, USA). The carrier gas was Helium with 99.999% purity (Roham gas Co. Tehran, Iran) at 1 ml/min flow rate. MS transfer line temperature was set at 220o C. A syringe pump (SEP-10S Plus, Aitecs, Lithuania) was used for introduction of the test analytes into the air flow. Syringe pump was calibrated prior to each injection according to the procedure described elsewhere.7 The diluents gas flow rate in the system was checked continuously by a dry gas meter (Elster-Handel, Germany) calibrated by primary standard. The temperature in the chambers was controlled by drawing air through heating coil actuated by thermocouple system with a temperature sensor (SAMWON ENG, Model SU-105, South Korea). Various air humidity rates were generated using an impinger system at different bubbling flows and temperatures. Air humidity in the system was continuously monitored by Testo 601 hygrometer (Model Testo 601, Testoterm GmbH & Co, Germany).
Results
System configuration
The original version of the system was constructed according to the findings and suggestions of other studies.8, 9 The system was designed in a way to take samples in different temperature, humidity, and velocity rates. Based on the proposed configuration, samples were taken from 5 different velocities. The chamber consisted of 6 sampling ports at its top for sampling under different velocities by SPME. Two other ports were also located on the rear section of the chamber which were suitable for use of sorbent tube samplers. The SPME sampling ports were equipped with GC septum to prevent any undesirable leakage from the chamber.
Analyte introduction
A CAR/PDMS SPME fiber was used as a passive sampler to study the adsorption isotherm of the coating or target analytes. Desired analytes were dissolved in carbon disulfide and then injected to the system. The concentration in the chamber was calculated by the equation 1 at 25° C.7
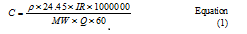
There are several techniques for preparation of test atmosphere in occupational exposure assessment studies. In general, these techniques can be divided into two main dynamic and static categories.1 However, there are numerous configurations which merge these techniques for generation of test atmosphere in specific cases.2 These techniques are reviewed extensively in the literature.3-5 Static techniques use a specified mass of analyte in a specific volume of a container for generation of test atmospheres. These methods are easy to prepare and cheap. But they also have some disadvantages such as: 1) adsorption of the analyte on the container walls, 2) non- continuous flow preparation, and 3) decrease of concentration due to withdrawal of a specific volume of mixture. Despite all of these negative points, it could be a good method in the case of using large containers and withdrawal of a small portion of this volume (less than 5%). Dynamic techniques deliver continuous mass of analyte to the continuous flow of diluent gas. These techniques can be divided into three general sub-groups of syringe injection, permeation tubes, and gas dilution.
Most techniques reviewed in different papers only described methods for preparation of specific concentration and did not deal with preparation of standard concentration at different environmental conditions. Therefore, preparation of a correct configuration for generating specific concentration of analyte in specific environmental conditions is another problem which must be taken into account. Selection of an appropriate method for generation of test atmosphere in occupational health studies is also another challenge and depends on the target concentration, nature of analyte, desirable environmental parameters, and the purpose of the study. Besides these, a good system must be qualified in other aspects, such as ability to generate a stable concentration in a prolonged time, and produce the desirable concentration in the various environmental conditions.
In this study, a hybrid system based on syringe injection and double dilution (stream dilution) for generation of test atmosphere was desired. The system configuration was set up for development of sampling methods based on passive samplers especially solid phase micro extraction (SPME).
Methods
Chemicals and materials
Carbon tetrachloride (99.5%), 1-butanol (99.5%), tetrachloroethylene (99%), and carbon disulfide (99%) were obtained from Merck (Darmstadt, Germany). USP grade Halothane was purchased from Nicholas Primal (Mumbai, India). SPME fibers included 100 µm poly-dimethylsiloxane (PDMS) and 75 µm carboxen/PDMS (CAR/PDMS) and manual holders were supplied from Supelco (Bellefonte, PA, USA).
Analysis
All new SPME fibers were conditioned in gas chromatography (GC) injector and desorbed according to the procedure described elsewhere.6 All SPME samples were analyzed with a Varian 3800 GC equipped with a Saturn 2200 ion trap mass spectrometer (MS). The GC was fitted with a 60 m×0.25 mm I.D and 1.5 μm film thickness capillary VOCOL column (Supelco, Bellefonte, USA). The carrier gas was Helium with 99.999% purity (Roham gas Co. Tehran, Iran) at 1 ml/min flow rate. MS transfer line temperature was set at 220o C. A syringe pump (SEP-10S Plus, Aitecs, Lithuania) was used for introduction of the test analytes into the air flow. Syringe pump was calibrated prior to each injection according to the procedure described elsewhere.7 The diluents gas flow rate in the system was checked continuously by a dry gas meter (Elster-Handel, Germany) calibrated by primary standard. The temperature in the chambers was controlled by drawing air through heating coil actuated by thermocouple system with a temperature sensor (SAMWON ENG, Model SU-105, South Korea). Various air humidity rates were generated using an impinger system at different bubbling flows and temperatures. Air humidity in the system was continuously monitored by Testo 601 hygrometer (Model Testo 601, Testoterm GmbH & Co, Germany).
Results
System configuration
The original version of the system was constructed according to the findings and suggestions of other studies.8, 9 The system was designed in a way to take samples in different temperature, humidity, and velocity rates. Based on the proposed configuration, samples were taken from 5 different velocities. The chamber consisted of 6 sampling ports at its top for sampling under different velocities by SPME. Two other ports were also located on the rear section of the chamber which were suitable for use of sorbent tube samplers. The SPME sampling ports were equipped with GC septum to prevent any undesirable leakage from the chamber.
Analyte introduction
A CAR/PDMS SPME fiber was used as a passive sampler to study the adsorption isotherm of the coating or target analytes. Desired analytes were dissolved in carbon disulfide and then injected to the system. The concentration in the chamber was calculated by the equation 1 at 25° C.7
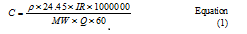
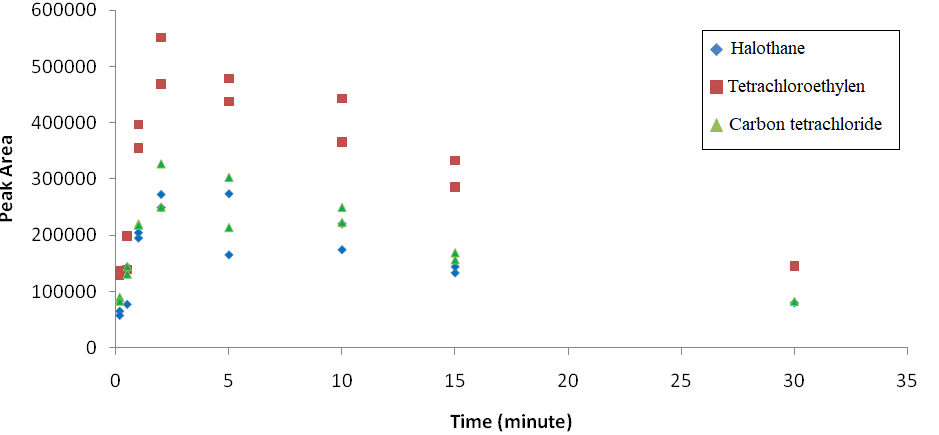
hereρ is the density of analyte (g/l), IR is the injection rate (ml/hr), MW is the molecular weight of analyte, C is the desirable concentration in chamber (ppm), and Q is the diluent gas flow rate (liter per minute). Effect of solvent constituent on derived isotherm is shown in figure 1. In a double dilution setup at the first stage, the target analytes were injected in their pure form to the first chamber. Based on the desired concentration, a small portion of the air stream in the first chamber (Q1) was pumped to the second one and diluted by the second stream (Q2). In the case of stream dilution, the concentration of the desired analyte in the second sampling chamber could be calculated by equation 2.

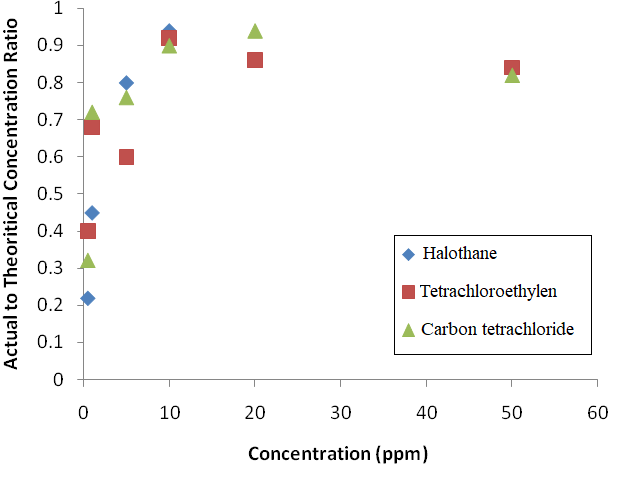
Where C is the concentration in the second chamber, C1 is the concentration in the first chamber, Q1 is the flow rate pumped from the first chamber, and Q2 is the diluent gas flow rate (liter per minute). Results showed that actual concentration in the chamber is different from the values calculated by the above equation (Figure 2).
Temperature control system
Heating section was placed in front of the analyte injection port to increase the volatilization of the analyte when introduced into the flow. The cross-section area of the air passing tube also was decreased to increase the turbulence around the needle. In order to prepare different air temperatures in the chamber, a heating system based on an incandescent lamp was constructed. Air was drawn to the lamp chamber and heated by the incandescent lamp. To control the temperature, a thermocouple and microcontroller was used. A sensor was later located in the chamber to sense the temperature. The sensed temperature logged to the microcontroller which was set in a specified temperature. The controller turned the lamp off in the case that the chamber temperature reached the pre-set value. When the temperature fell below the pre-set value, the microcontroller turned the lamp on. This system can produce a temperature controlled condition after about 10 minutes from the start-up with ± 0.3° C error.
Humidity
Humidity generation was studied in the range of 30 -80%. Humidification was performed based on water vapor addition to the air flow. The level of humidification in the system was controlled by temperature, depth of water impingement in the flask, and the flow rate of incoming current. Findings showed that for high humidity levels (above 30 %) it is necessary to use a chamber with elevated temperature in its walls. Therefore, an anti-condensation coil was constructed and placed in the system.
Anti-condensation system
Another temperature system was applied in the outer wall of the chamber to avoid condensation in humidity conditions. Results showed that in preparation of high humidity content conditions (80% and above), the water vapor will condensate on the inner walls of the chamber. We applied a heating coil around the chamber to heat the chamber's wall and prevent the condensation of the water droplets on the surface of the chamber. The heating power of the heating oil was adjustable, so it was set at 5° C higher than the system's temperature for each temperature condition. After application of a system, the results indicated that repeatability improved significantly in generation of test atmosphere in high humidity conditions.
Velocity
Two systems were used for preparation of various air velocities in the chamber. A system was initially designed for production of velocity in the range of 0.05 - 0.5 m/s. Different velocities were prepared by changing the cross-section area of each section of the chamber.
Validity
Time to reach the steady-state (tss) for desired concentration was studied in the chamber. The tss was studied by taking samples during 2 hours (every 5 minutes) through an exposed 100 µm PDMS SPME fiber. We found that the concentration reached the steady state shortly after the beginning of injection. However, the concentration in the beginning of system startup was significantly lower than the values observed for tss. Results further indicated that with decrease in analyte concentration in the chamber, the tss gets longer. The repeatability of the system was also checked by calculating the relative standard deviation (RSD) of the measurements. Table 1 represents the results of validation for compounds of interest. Statistical comparison of the results showed that there is no significant difference between the RSD values observed in different temperature and humidity rates for the desired compounds.
Discussion
Results of this study showed that injection of desired analyst in the form of liquid dissolved in another solvent as a diluent (in this study carbon disulfide) leads to occurrence of a huge error in obtaining appropriate adsorption isotherm. Most of the passive sampling media were composed of adsorbent materials. One of the most important characteristics of adsorbent materials is their capacity. Loading the adsorbent with a mass higher than its capacity leads to breakthrough. The competition between analytes for occupying the active sites of the porous sorbent is also common and occurs in the form of sampler saturation and analyst loss.10 In order to solve this problem, the system configuration shifted to a double dilution system which consisted of two cascade analyte introduction parts. It is a relatively large deviation from the concentration predicted by equation in the low concentration, but with the increase of the concentration, the ratio of observed to the calculated concentration increased. It seems that factors such as adsorption on the wall of container lead to concentration decay in the chamber. However, using equations to predict the concentration leads to some degree of error, but it can be used as a guideline at the beginning of the system start-up. The concentration should then be measured precisely by active sampling and analysis based on standard methods. A comprehensive study on air velocity measurement in workplaces showed that about 85% of work environments have an air velocity below 0.35 m/s.11 Our chamber was capable to produce a test atmosphere in this range and therefore is applicable for studies aimed to simulate work environments. However, the other configurations similar to the designed chamber in our study previously used across studies.9 According to this design, the velocity will be changed according to the law of mass conservation. Examination of the system tss was necessary because taking samples in the time-span before tss, leads to inaccurate results. Active adsorption sites on the chamber walls and tubing seems to be responsible for the concentration change during start-up. Small tss in the study shows that saturation of adsorption sites over a short time after the beginning of system operation inhibits reduction of concentration in the chamber.
In order to prepare known concentrations of gaseous analytes in the desired range, a dynamic atmosphere generation system was built in the laboratory. Results showed that it is possible to create a wide range of concentration with the described system by changing the parameters of diluent flow and injection rates. Application of double dilution system leads to better concentration preparation in cases which the injection rate of the syringe is the limiting factor. Theoretically, it is possible to create sub ppb concentration in the chamber with this configuration, while, the lowest optimum concentration seems to be not below 1 ppb. Due to adsorption of the analytes on the wall of the chamber and poor repeatability, this system is not recommended to be used for preparation of the concentrations lower than 1 ppb. Results indicated that with lowering the concentration, the stabilizing time for concentration increases exponentially. However, 50 minutes seems to be sufficient to have the highest efficiency. Results of the repeatability test showed that the system repeatability is good (RSD < 5%) even in cases with elevated temperature and humidity.
Temperature control system
Heating section was placed in front of the analyte injection port to increase the volatilization of the analyte when introduced into the flow. The cross-section area of the air passing tube also was decreased to increase the turbulence around the needle. In order to prepare different air temperatures in the chamber, a heating system based on an incandescent lamp was constructed. Air was drawn to the lamp chamber and heated by the incandescent lamp. To control the temperature, a thermocouple and microcontroller was used. A sensor was later located in the chamber to sense the temperature. The sensed temperature logged to the microcontroller which was set in a specified temperature. The controller turned the lamp off in the case that the chamber temperature reached the pre-set value. When the temperature fell below the pre-set value, the microcontroller turned the lamp on. This system can produce a temperature controlled condition after about 10 minutes from the start-up with ± 0.3° C error.
Humidity
Humidity generation was studied in the range of 30 -80%. Humidification was performed based on water vapor addition to the air flow. The level of humidification in the system was controlled by temperature, depth of water impingement in the flask, and the flow rate of incoming current. Findings showed that for high humidity levels (above 30 %) it is necessary to use a chamber with elevated temperature in its walls. Therefore, an anti-condensation coil was constructed and placed in the system.
Anti-condensation system
Another temperature system was applied in the outer wall of the chamber to avoid condensation in humidity conditions. Results showed that in preparation of high humidity content conditions (80% and above), the water vapor will condensate on the inner walls of the chamber. We applied a heating coil around the chamber to heat the chamber's wall and prevent the condensation of the water droplets on the surface of the chamber. The heating power of the heating oil was adjustable, so it was set at 5° C higher than the system's temperature for each temperature condition. After application of a system, the results indicated that repeatability improved significantly in generation of test atmosphere in high humidity conditions.
Velocity
Two systems were used for preparation of various air velocities in the chamber. A system was initially designed for production of velocity in the range of 0.05 - 0.5 m/s. Different velocities were prepared by changing the cross-section area of each section of the chamber.
Validity
Time to reach the steady-state (tss) for desired concentration was studied in the chamber. The tss was studied by taking samples during 2 hours (every 5 minutes) through an exposed 100 µm PDMS SPME fiber. We found that the concentration reached the steady state shortly after the beginning of injection. However, the concentration in the beginning of system startup was significantly lower than the values observed for tss. Results further indicated that with decrease in analyte concentration in the chamber, the tss gets longer. The repeatability of the system was also checked by calculating the relative standard deviation (RSD) of the measurements. Table 1 represents the results of validation for compounds of interest. Statistical comparison of the results showed that there is no significant difference between the RSD values observed in different temperature and humidity rates for the desired compounds.
Discussion
Results of this study showed that injection of desired analyst in the form of liquid dissolved in another solvent as a diluent (in this study carbon disulfide) leads to occurrence of a huge error in obtaining appropriate adsorption isotherm. Most of the passive sampling media were composed of adsorbent materials. One of the most important characteristics of adsorbent materials is their capacity. Loading the adsorbent with a mass higher than its capacity leads to breakthrough. The competition between analytes for occupying the active sites of the porous sorbent is also common and occurs in the form of sampler saturation and analyst loss.10 In order to solve this problem, the system configuration shifted to a double dilution system which consisted of two cascade analyte introduction parts. It is a relatively large deviation from the concentration predicted by equation in the low concentration, but with the increase of the concentration, the ratio of observed to the calculated concentration increased. It seems that factors such as adsorption on the wall of container lead to concentration decay in the chamber. However, using equations to predict the concentration leads to some degree of error, but it can be used as a guideline at the beginning of the system start-up. The concentration should then be measured precisely by active sampling and analysis based on standard methods. A comprehensive study on air velocity measurement in workplaces showed that about 85% of work environments have an air velocity below 0.35 m/s.11 Our chamber was capable to produce a test atmosphere in this range and therefore is applicable for studies aimed to simulate work environments. However, the other configurations similar to the designed chamber in our study previously used across studies.9 According to this design, the velocity will be changed according to the law of mass conservation. Examination of the system tss was necessary because taking samples in the time-span before tss, leads to inaccurate results. Active adsorption sites on the chamber walls and tubing seems to be responsible for the concentration change during start-up. Small tss in the study shows that saturation of adsorption sites over a short time after the beginning of system operation inhibits reduction of concentration in the chamber.
In order to prepare known concentrations of gaseous analytes in the desired range, a dynamic atmosphere generation system was built in the laboratory. Results showed that it is possible to create a wide range of concentration with the described system by changing the parameters of diluent flow and injection rates. Application of double dilution system leads to better concentration preparation in cases which the injection rate of the syringe is the limiting factor. Theoretically, it is possible to create sub ppb concentration in the chamber with this configuration, while, the lowest optimum concentration seems to be not below 1 ppb. Due to adsorption of the analytes on the wall of the chamber and poor repeatability, this system is not recommended to be used for preparation of the concentrations lower than 1 ppb. Results indicated that with lowering the concentration, the stabilizing time for concentration increases exponentially. However, 50 minutes seems to be sufficient to have the highest efficiency. Results of the repeatability test showed that the system repeatability is good (RSD < 5%) even in cases with elevated temperature and humidity.
Table 1. Repeatability of the measurements in the atmosphere generation system based on the observed relative standard deviation (RSD) for repeated measurement by SPME at 1 ppm of selected analytes (the effect of sampler variability excluded).
| Analyte | Humidity (%) | Temperature (º C) | Velocity(m/s) | |||||||
| 30 | 50 | 80 | 20 | 25 | 30 | 0.05 | 0.10 | 0.30 | 0.50 | |
| Halothane | 2.68 | 2.96 | 1.23 | 5.55 | 1.80 | 6.02 | 2.53 | 3.72 | 2.14 | 1.25 |
| Tetrachloroethylene | 4.57 | 3.24 | 3.97 | 2.78 | 0.87 | 2.34 | 5.59 | 2.58 | 6.74 | 2.23 |
| Carbon tetrachloride | 1.77 | 4.60 | 4.95 | 3.43 | 2.25 | 3.49 | 3.82 | 3.05 | 3.84 | 4.44 |

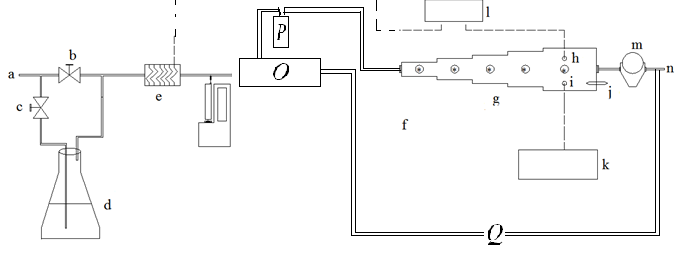
Figure 1. Schematic diagram of test atmosphere generation system.
a: Air inlet, b: Bypass valve, c: Valve to humidity system, d: Humidity generation system, e: Electrical coil, f: Syringe pump, g: Sampling chamber, h: Temperature sensor, i: Humidity sensor, j: Active sampling port, k: Hygrometer, l: Thermocouple, m: Dry gas meter, n: Outlet (to hood), *: Septum equipped sampling ports

Figure 2. Effect of solvent (carbon disulfide) on derived isotherm of halothane, tetrachloroethylene, and carbon tetrachloride.

Figure 3. Effect of analyte concentration on applicability of equation for prediction of actual concentration
in the test atmosphere generation system
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
The authors wish to extend appreciate to Hamadan University of medical sciences for financial support of this study.
References
1. Konieczka P, Namieśnik J, Biernat JF. Generation of standard gaseous mixtures by thermal decomposition of surface compounds: Standard mixtures of thiols. Journal of Chromatography A. 1991;540:449-55.
2. Greenhouse S, Andrawes F. Generation of gaseous standards using exponential dilution flasks in series. Analytica Chimica Acta. 1990; 236: 221-6.
3. Barratt R. The preparation of standard gas mixtures. A review. Analyst. 1981;106(1265):817-49.
4. Naganowska-Nowak A, Konieczka P, Przyjazny A, Namieśnik J. Development of techniques of generation of gaseous standard mixtures. Critical reviews in analytical chemistry. 2005;35(1):31-55.
5. Namiesnik J. Generation of standard gaseous mixtures. Journal of chromatography. 1984;300:79-108.
6. Zare Sakhvidi MJ, Bahrami AR, Ghiasvand A, Mahjub H, Tuduri L. Determination of Inhalational Anesthetics in Field and Laboratory by SPME GC/MS. Analytical Letters. 2012;45(4): 375-85.
7. Pisaniello D. The generation of test atmospheres for occupational hygiene laboratory evaluation of organic vapour monitoring devices: report prepared for the Occupational Health and Radiation Control Branch;2008.
8. Lee IS, Tsai SW. Passive sampling of ambient ozone by solid phase microextraction with on-fiber derivatization. Analytica Chimica Acta. 2008; 610(2):149-55.
9. Koziel JA, Martos PA, Pawliszyn J. System for the generation of standard gas mixtures of volatile and semi-volatile organic compounds for calibrations of solid-phase microextraction and other sampling devices. Journal of Chromatography A. 2004; 1025(1): 3-9.
10. Senum GI. Theoretical collection efficiencies of adsorbent samplers. Environmental Science & Technology. 1981;15(9): 1073-5.
11. Baldwin PEJ, Maynard AD. A survey of wind speeds in indoor workplaces. Annals of Occupational Hygiene. 1998;42(5):
303-13.
The authors declare no conflict of interest.
Acknowledgement
The authors wish to extend appreciate to Hamadan University of medical sciences for financial support of this study.
References
1. Konieczka P, Namieśnik J, Biernat JF. Generation of standard gaseous mixtures by thermal decomposition of surface compounds: Standard mixtures of thiols. Journal of Chromatography A. 1991;540:449-55.
2. Greenhouse S, Andrawes F. Generation of gaseous standards using exponential dilution flasks in series. Analytica Chimica Acta. 1990; 236: 221-6.
3. Barratt R. The preparation of standard gas mixtures. A review. Analyst. 1981;106(1265):817-49.
4. Naganowska-Nowak A, Konieczka P, Przyjazny A, Namieśnik J. Development of techniques of generation of gaseous standard mixtures. Critical reviews in analytical chemistry. 2005;35(1):31-55.
5. Namiesnik J. Generation of standard gaseous mixtures. Journal of chromatography. 1984;300:79-108.
6. Zare Sakhvidi MJ, Bahrami AR, Ghiasvand A, Mahjub H, Tuduri L. Determination of Inhalational Anesthetics in Field and Laboratory by SPME GC/MS. Analytical Letters. 2012;45(4): 375-85.
7. Pisaniello D. The generation of test atmospheres for occupational hygiene laboratory evaluation of organic vapour monitoring devices: report prepared for the Occupational Health and Radiation Control Branch;2008.
8. Lee IS, Tsai SW. Passive sampling of ambient ozone by solid phase microextraction with on-fiber derivatization. Analytica Chimica Acta. 2008; 610(2):149-55.
9. Koziel JA, Martos PA, Pawliszyn J. System for the generation of standard gas mixtures of volatile and semi-volatile organic compounds for calibrations of solid-phase microextraction and other sampling devices. Journal of Chromatography A. 2004; 1025(1): 3-9.
10. Senum GI. Theoretical collection efficiencies of adsorbent samplers. Environmental Science & Technology. 1981;15(9): 1073-5.
11. Baldwin PEJ, Maynard AD. A survey of wind speeds in indoor workplaces. Annals of Occupational Hygiene. 1998;42(5):
303-13.
Type of Study: Research |
Subject:
Special
Received: 2017/10/11 | Accepted: 2017/10/11 | Published: 2017/10/11
Received: 2017/10/11 | Accepted: 2017/10/11 | Published: 2017/10/11
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





